Nickel (Ni) is a strategic and hard-to-ripeless element used in 1.97 million tonnes of stainless steel and 210 kilotons non-Ferrus Mishra, especially superloy, annually.1,23Both alloy families indirectly serve stability, through the increased longevity of the products in the east and through high efficiency of the engine. However 70% of the current global annual NI production (3 million ton)1,23 Luck for the stainless-steel sector is, the decarbonization of the transport sector through the use of ni-based battery electrodes in electric vehicles requires a completely additional 3 million tons of ni for battery production by 2040.1,2,34 (Fig. 1A). Currently, 60% of the annual NI production depends on high-grade sulfide ores (with 1.5–4 WT% ni content). Low-grade ore variants, namely, letters (with an average 1.5 WT% ni content), which are divided into two variants, namely, seprid and limonite, the remaining 40% (providing the referee. 8Fig. 1 b). However, land-based NI reserves are inversely distributed, namely, 60% of the total NI available in nature is found in letterites, and only 40% in sulfide ores8 (Fig. 1b).
Comparison with market growth, source, production, processing passage, emission and permanent one-phase hydrogen-plasma route. ACurrent and estimated (2040) Ni-Market growth, extending the demand for the battery electrode precursor material required for electrification of the transport sector, demanding NI to 6 million tonnes (data from the referring. 1), On a staggering environmental toll of 20 tons co -co -cum2 Per ton of NI (10 times of emissions due to the production of a ton of steel). BPailed land-based world NI-resources distribution and global NI supply (Data from Ref. 8, CAssociate2 Emission from various extraction routes (tons per ton of ni). The inner and external circles reflect the lower and upper emission boundaries. The highlighted middle ring number reflects the weighted average for each process (data from Ref. 15Show bar plots on the right overall average CO2 Other industries (data from referee. 5,7, DCurrent processing routes and comparative analysis of HPSR: The planned diagram highlights separate processing stages for each extraction method. The One-step HPSR stands out as a single-phase process, directly feeds dried ore in a furnace filled with floods with an AR-H2 Mixing. This streamlined process products produce high -grade ferronicale, slag and gaseous water.
In sulfidic ore deposits, Ni is mainly present as NIS, NI, as NIS, Ni-rich minerals2Faiz4 And (cum, ni)3S4Chemical simplicity of sulfide minerals enables effective separation of gangu impurities from valuable ni-bearing compounds using traditional techniques such as froth plotions.9However, the finite reserves of Nee Sulfides cannot meet rapidly growing global NI demand, requiring permanent NI production from abundant, lower-grain letters. In letterite deposits, Ni is not found in the form of discrete minerals; It dissolves within complex magnesium (mg) -Silicates (namely, siprolytes)10Such as (milligrams, f, ni)3Sai2Hey5(Oh)4 And (mg, p, ni)3Sai4Hey10(Oh)2.4h2O, or partially replaces iron (Fe) in Fe-Oxide Lattices (Limonite)7Namely, Goethite (Fe, Ni) Ooh. This mineral and chemical complexity limits the efficient and sustainable benefits of these ores to the downstream green technologies with ni-mentor substances. This fundamental challenge inspired us to develop a completely different approach, namely, the process of smelting the entire dry ore charge in the same metallurgy step using hydrogen plasma. This process integrates calculation, smelting and purification at the same stage, with all these operations within a furnace, thus, only, allows direct exploitation of high-graded ferronicale from dried ore charge in a metal phase. A detailed description of the term ‘single metalgical step’ is provided in the supplementary information section ‘Definition of single metallurgical phase’.
Current industrial ne-tectrate processing routes are largely determined by the crystallographic structure and ore of ni and FE content of ni-hosting stages. Characterized by Limonite ore, cum and MGO content (<4 WT% Mg), usually processed through high pressure acid leaching (HPAL) for NI and Cobalt (CO) recovery, when present, when present,11,12Conversely, silicate-rich saprolytes, which are difficult to reduce, undergo pyrometalgical processing. It is known as the RK-EF, after the ore drying in the ore drying and partial decrease in the rotary kiln (RK), in the electric arch furnaces (EAFS), using coke as coke.13,14Or optionally in blast furnace13Further technical details are provided in the current industrial processing route of the supplementary information section ‘Ni-Teterites’
The demand for energy for lettering-OR processing is very high, with about 570 grams per tonne to about 570 grams per tonne to about 570 grams of GJ per tonne. 5,6), More than 22 GJ per tonne required for steel. Greenhouse gas emission is also sufficient, 45 ton carbon dioxide equivalent (CO) with RK-EF/blast furnace and HPAL routes2E) per tonn and 14 tonnes cum2E per tonne NI, respectively15 (Fig. 1 c). However, 60% of NI comes from low-ecosion sulfide processing (6 tons).2E per tonne)15Letters are responsible for 40%, pushing the overall footprint of the industry to about 20-27 tonnes of cum2E per tonne ni (refuses. 5,67Fig. 1 C), more than 10 times from steel (2.3 ton CO2E per ton). This makes NI one of the most environmental harmful metals to produce (Fig. 1 C).
To reduce emissions, researchers have examined the replace of carbon-based rectants with hydrogen gas (H)2,7 Solid-state of letters in direct decrease, especially limonite7,16,17However, complex crystallographic structure, low ni material (0.5-2 WT%) and 90% impurity material (silicon (si), calcium (CA) and aluminum (aluminum (AL) to make some names), leading to some names), leading to dull cannteix.16,18 And disabled hydrogen usage18,19And many additional pre -pre -pre -treatment is required20 To separate the metal from oxide. Direct reduction of septions with gaseous hydrogen2) Rarely reported, perhaps due to thermodynamic stability of silicates18,21 Typical direct-cutting temperature (800–1,000 ° C), and its deficiency by molecular hydrogen using catalytic compounds cannot be possible without pre-chemical changes in simple substances like NIOs22Supplementary Information Section ‘Solid-State Dr. The letter is wide in ‘Letters’.
To remove these boundaries, especially people associated with saprolytes, we are subject to the ore (extended data tables 1 and 2) for single-phase hydrogen-plasma molten state process, causing christallographic composition of minerals (for example, (MG, FE, NI)3Sai2Hey5(Oh)4Fig. 2b) In simple ionic species (FE)2+Ni2+MG2+O!2- And sio44)) Without the need of catalysts, and concomitantly exposing it to highly reactive hydrogen-plasma species (H)2H and H,This approach consolces long and environmentally harmful processes shown in figs into a single metallurgy phase: hydrogen-plasma smalting reduction (HPSR), beneficial, metal separation and purification. Pulled on fully renewable energy, it replaces carbon-based fuel and redeemation with renewable electricity and hydrogen, which offers 18% energy savings and decrease in CO2 Emission of up to 84%. Additional plasma-free use with melted nea ore and molecular hydrogen was conducted as reference and comparison tests. The results showed quite slow reaction to kinetics with molecular hydrogen compared to hydrogen plasma, discussing in detail the supplementary information section ‘experiments with molecular hydrogen’.
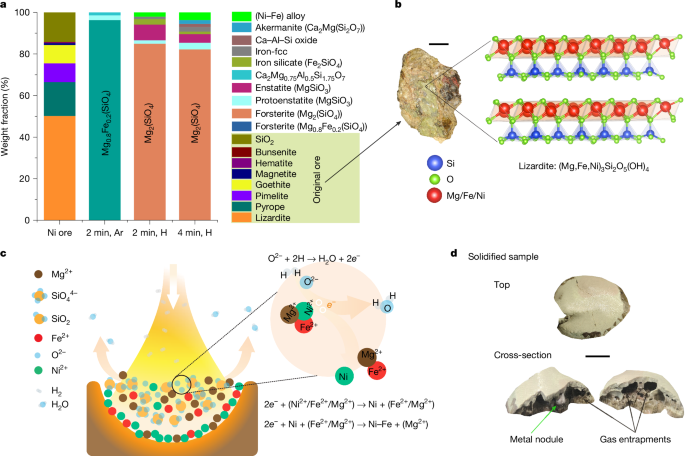
Snapshot, a visible ni-hosting mineral structure, deficiency mechanism and a visible snapshot of a solid sample in the original ore. APhase development during melting of original ore in AR and a lean H2 Environment (AR -10% h)2) For 2 minutes and 4 minutes, simple MG-cylocated stages reflect the change of complex original ore with different stages. The word ‘(Ni-fe) alloy’ (shown in bright green) represents metal nodules, and ‘Iron-FCC’, shown in gray, indicates iron drops complicated in silicates that cannot be mechanically recovered. BThe picture of the original ore with the complex structure of the lizard which is present in it. The lizard displays the ability to host Ni2+/Fe2+ Ion instead of milligram2+ To highlight structural complications within ions, ore. CPlanned hypothesis of shortage system. The rainfall of metals is started by removing free oxygen from melting. Hydrogen species extract free oxygen, leaving two electrons behind. These electrons select reacts with Ni2+To inspire the rainfall of metal ni. This ionic reactions have also been illustrated. DSolid sample and cross-section. In particular, metal nodules are observable within samples, as well as with adequate porosity as a result of the entry of gas. Scale bar, 1 cm (B) And 5 mm (D,
Our probe focuses on understanding hydrogen-plasma deficiency behavior of somolitic low-grade ni letterite ore (extended data tables 1 and 2). This approach marks a promising departure from traditional industrial techniques such as RK-EF/blast furnace and HPAL, carbon (C)-and sulfur (s)-bed by changing or eliminating the direct CO2 And sulfur dioxide (so2) Emission, ignoring the use of harmful acids (for example, sulfuric acid (H)2so4) In HPAL), and denying the need for expensive east and post-treatment (Image 1D). This study provides experimental evidence supporting one-step HPSR as a permanent option for metal production from both oxide and silicates. It extends feedstock options to low cost, low-grade minerals. We examine the response mechanisms of the extraction from thermodynamics, chemical partitions, microstic evolution, and letritic ores, applying these principles to accurate and optimal procedure design.


