A new extended prostate cancer drug can bring new hope for patients with a common form of the disease.
Switzerland -based pharmaceutical company, Novartis, on March 28 announced that the US Food and Drug Administration (FDA) has expanded the approval for Pluvikto (Lutetium Lu 177 Vipotide Tetraxate) for a target radioligand therapy (RLT) which has been given before chemotherapy.
(RLT is a form of targeted nuclear therapy that uses to treat many types of cancer according to Doctor Novartis.)
The risk of prostate cancer increases by 45% that shares a troubled behavior
This drug is for patients with PSMA positive metastatic caustation-resistant prostate cancer (MCRPC), who have found a square of drugs used in the treatment of metastatic prostate cancer, a square androgen receptor Pathway innial (ARPI).
A new extended prostate cancer drug can bring new hope for patients with a common form of the disease. (Istock)
According to the Novartis press release, Pluvulto first received the FDA approval on 23 March 2022, but this new extended approval tries the number of qualified patients to obtain the drug.
The drug is administered in the bloodstream through an IV, where it connects to prostate cancer cells and either prevents them from replica or killing them.
Cases of prostate cancer share the potential reasons as spikes in this US state
The first indication for Pluvulto for patients with Michael Morris, MD, MD, the head of the prostate cancer section at the Memorial Slone Catering Center in New York, can actually change our remedies for MCRPC patients. ,
“It provides a targeted therapy that delays the progression of better disease than another ARPI. This approval is an important step and should open the door for a therapy, with MCRPC with clear clinical benefits for the patient with MCRPC that has progressed on an ARPI and not receiving chemotherapy.”
In clinical trials, Pluvulto “reduced the risk of progress or death by 59%”.
It is a form of prostate cancer that has spread to other parts of the body and according to webmD, standard hormone does not respond to therapy.
It also contains high levels of prostate-specific membrane antigens (PSMA), a protein produced by prostate cancer cells.
In clinical trials, Pluvicto, up to 59% of MCRPC patients, “reduced the risk of progress or death”, Novartis said.
image
“The extended approval of the FDA (Luttium LU 177 Vipotide tetraxeton) marked a transformative step in the treatment of MCRPC, underlining the increasing effect of priced oncology,” George A. Garcia, MD, is a genitorinary medical oncologist and president of solid tumor, in the university, who is from University Hospital cancer, solid tumnologist and president of solid tumor.
Click here to get Fox News app
“By enabling access to this targeted radioligand therapy before chemotherapy, we are not only wider treatment options, but also re -define the standard of care for PSMA positive disease.”
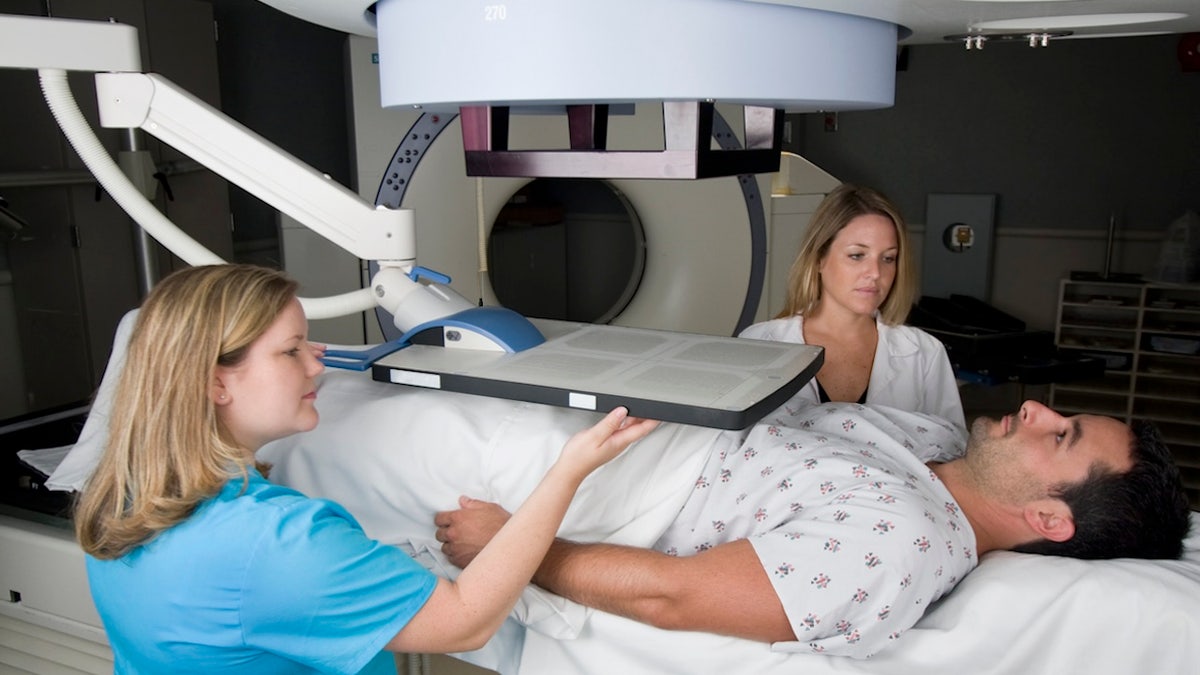
A researcher said, “By enabling access to this targeted radioligand therapy before chemotherapy, we are not only wider treatment options, but also re -define the standard of care for PSMA positive disease,” a researcher said. (Istock)
Prostate cancer is the second major cause of death in men; MCRPC creates 20% in most deaths and all metastatic prostate cancer cases.
Studies have shown that MCRPC develops within five years after initial treatment in about 10% to 20% of patients with prostate cancer, and matters of metastatic patients have increased by 4% to 5% every year since 2011.
Click here to sign up for our health newspaper
According to the American Cancer Society, sixty percent prostate cancer is diagnosed in men aged 65 or older. The risk of diagnosis of metastatic prostate cancer is usually between 65 and 74.
Adverse effects of Pluvulto include dry mouth (61%), fatigue (53%), nausea (32%) and constipation (22%).
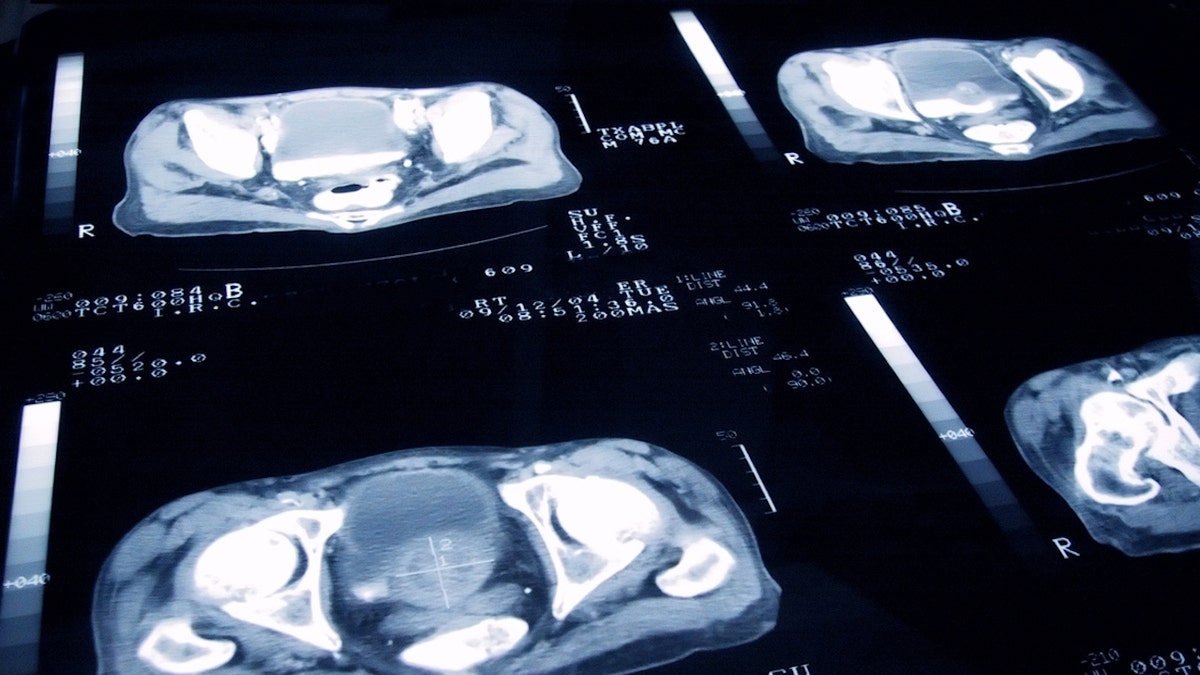
Prostate cancer is the second major cause of death in men; MCRPC creates 20% in most deaths and all metastatic prostate cancer cases. (Istock)
The patients receiving the drug were able to proceed with chemotherapy after taking it.
The company said that Novartis is committed to giving pluvicto to about 600 RLT treatment sites in the US.
For more health articles, travel www.foxnews.com/health
Further, Novartis stated that it plans to check the use of RLT for other types of advanced cancer including breasts, colon, neuroendocrine, lungs and pancreatic cancer.


